How Do Electrons in the Same Atom Differ
What is different is the number of neutrons The different number of neutrons all cause a difference in the atomic weight or mass of the atoms. Use the periodic table to determine which atom would have similar chemical properties to this atom.
Atoms Isotopes Ions And Molecules Boundless Biology
Which of the following is Bronsted-Lowry acid from the following reaction.
. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. In atoms hyperfine structure arises from the energy of the nuclear magnetic dipole moment interacting with the magnetic field generated by the electrons and the energy of the nuclear electric quadrupole moment in the electric field gradient due to the distribution of charge within the atom. They move up and down energy shells between orbitals and energy levels.
Protons and neutrons are found in the nucleus of an atom while electrons are found in the area surrounding the nucleus of an atom. Protons have a positive charge electrons have a negative charge and neutrons have no charge. Electrons occupy different energy levels or shells.
This is important in the formation of ionic compounds and covalent compounds. The proton and the neutron are present in the nucleus of the atom whereas the electrons are at the periphery of the atom. Basically the concepts express the tendency for electrons within molecules to seek out the lowest energy configurations possible electrons occupy the lowest energy orbitals within an atoms shells.
We refer to the atoms of the same element with different numbers of neutrons as isotopes. Protons are less mobile as compared to electrons. Electrons - negatively charged.
A They differ in the number of electrons b They differ in the number of protons c They differ in the number of neutrons d None of these 10. Two electrons occupying the same orbital in the same atom must have opposite spins - one spins clockwise the other counterclockwise. We can easily remove electrons from an atom.
Thats an atom in a nutshell. The number of protons in an atom of an element is the elements BLANK BLANK Protons. An ion has an unequal number of protons and electrons.
If you look at a table of ionization energies you will see that each element binds its electrons with different energies and as shown by hydrogen and deuterium there is even a slight dependency on mass. How do two sublevels of the same principal energy levels differ from each other. Each energy sublevel corresponds to an orbital of a different shape which describes where the electron is likely to be found.
A neutral atom has the same number of protons and electrons charges cancel each other out. Click to see full answer. Since the attraction between valence electrons and the nucleus of an atom is less valence electrons can easily be removed than the electrons in the inner orbitals.
Most of the mass of an atom is in the BLANK Atomic Number. The nucleus is stationary in orientation having proton and neutron in it whereas the periphery as the electrons are moving. The same chemical element can have a different number of neutrons and still be the same element.
The difference in the ratio between the number of protons the number of protons stay the same and the number neutrons. They differ in the number of neutrons. The atomic number is equal to the number of protons inside the atomic neucleus.
OCla HO b HO d OH HOCla OHaq a OCI c HOCL Answers 12345678910 11. Protons neutrons and electrons all have about the same mass. The electrons inside atoms can only have certain energies so they have what are called energy levels.
Isotopes are atoms with the same number of protons but a different number of neutrons inside the atomic nucleus. The nucleus of an atom contains neutrons and protons bonded tightly together. A12 protons 12 neutrons and 12 electrons B11 protons 11 neutrons and 11 electrons C10 protons science.
Attraction to Electric Field. If the charge is positive there are more protons than electrons. Click to see full answer.
The number of electrons is always equal to the the number of protons. How do an atom and an ion of the same element differ. Because the lithium atom loses a negatively charged electron it becomes a positively charged ion or cation.
Think of this as similar to the way the planets all orbit the sun. How do an atom and an ion of the same element differ. Protons and neutrons have about the same BLANK Electrons.
The protons and neutrons are in the atomic nucleus of the atom while the electrons orbit the atomic nucleus. Moving through the elements. Sodium atom is neutral whereas sodium ion is a charged specie with a charge of 1.
The number of protons and electrons in sodium atom is same ie 11 whereas the number of protons in sodium ion 11 is more than number of electrons 10. Which statement about subatomic particles is true. Well if a nutshell was.
The number of electrons dictates the atoms chemical behaviour. They differ in the number of electrons. For the best answers search on this site httpsshorturlimZ6xTd.
Chemistry questions and answers. How do you know if two atoms are the same element. Apart from their numbers the only real difference is the energy of the interactions between them.
Electron pair configuration VSEPR and molecular shapes go hand in hand with one another. Because the electron structure is the same isotopes have the same chemical properties. Valence electrons are responsible for chemical reactions and chemical bonding of an atom.
BLANK have much less mass than the other two particles in an atom. The basic difference between the particles of the atom are their charges and their orientation in the atom. How do two electrons in the same atom differ.
Each shell can hold a maximum number of electrons. Protons get attracted towards the Negative-ve side. The size of sodium ion is smaller than sodium atom.
Maybe they can absorb a certain amount of energy or twice that amount or two and a half times that amount changing in discrete amounts. Electrons are very fast-moving it mostly depends on the outer orbit of the atom the conductivity of the metal also depends on the flow of electrons in an atom. How can electrons in an atom move from one energy level to another.
They differ in the number of protons.

The Atom Chemistry Classroom Chemistry Help Electron Configuration
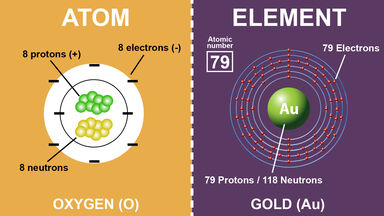
Difference Between Atoms And Elements With Examples

Copper Bohr Model What Is Electricity Electronics Projects Diy Electricity

Comments
Post a Comment